Designed for faculty, scholars, and staff
Research Education’s RECR certificate addresses all federal agency requirements, which includes topics such as rigor and integrity, ethical standards for peer review, protection of proprietary information and intellectual property, respectful treatment of students and colleagues, and research mentoring.
Most funding agencies, including NSF and NIH, request language in grant applications that reflects an understanding of RECR topics, such as Mentor Training Plans and other Supplementary Documents. Therefore, it can be helpful to complete RECR training even before you begin writing a grant application.
REQUIRED COURSES
| Foundations of Responsible Conduct of Research (RCR) - Faculty & Staff |
Self-Paced Online
|
| Case Studies in the Responsible Conduct of Research (RCR) |
Live Online
|
| Research Participant Advocacy and the Informed Consent Process |
Live Online
|
| Introduction to Research Mentoring |
Self-Paced Online
|
| Research Security Training |
Self-Paced Online
|
ELECTIVE COURSES (SELECT 1)
| Advanced Consideration of the Criteria for IRB Approval of Research OR |
Live Online
|
| Advanced Consideration of the Criteria for IRB Approval of Research |
Self-Paced Online
|
| Advanced Consideration of Reportable Events in Human Subject Research |
Live Online
|
| Conflict of Interest (COI) |
Self-Paced Online
|
| Collaborative Research and the Roles of the Scientist in Society |
Live Online
|
| Foreign Influence - Heightened Awareness and Increased Activity |
Self-Paced Online
|
| Getting Published: Responsible Authorship and Peer Review |
Self-Paced Online
|
| Informed Consent: Models and Requirements |
Live Online
|
| Laboratory Leadership and Staffing |
Self-Paced Online
|
| Preparation of Investigatory-Initiated Drug and Device Studies |
Self-Paced Online
|
| Introduction to Research Data Management |
Hybrid
|
| Understanding IRB Report Form Submissions in ERICA |
Self-Paced Online
|
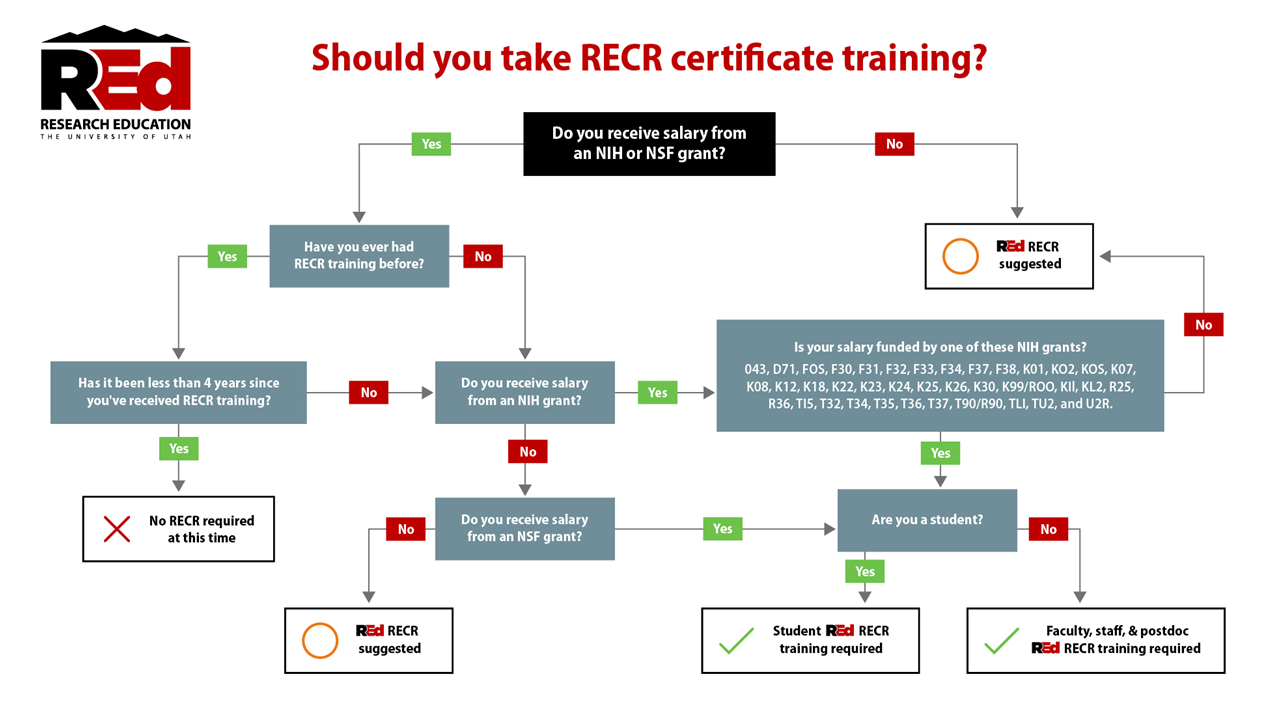
Do you need to take RECR training?
Responsible and Ethical Conduct of Research (RECR), also known as RCR, training is required for individuals who receive compensation from federally funded research grants, including those from:
- National Science Foundation (NSF)
- National Institutes of Health (NIH)
- U.S. Department of Agriculture (USDA)
- Other federal agencies
Training must typically be completed:
- Within 90 days for online formats
- Within 12 months for classroom formats
- Refresher training is required every three years for NIH-funded researchers
Completing RECR training ensures compliance with federal mandates and strengthens your understanding of research integrity, mentoring, peer review ethics, and more.