build a foundation for research success
With Classes from Research Education
See upcoming classes on our calendar.
Schedule a class for your group.
Class Catalog
Explore free, expert-led classes that help faculty, staff, postdocs, and students
strengthen their skills in key areas of research practice.
You can also visit our Canvas Catalog to search, register, and track your progress toward certificates of completion.
General Research Education (300-399)
| Managing and Maintaining Your Scholarly Profile |
Live Online
|
| Introduction to Research Data Management |
Hybrid
|
| Image Manipulation: Research Misconduct |
Live Online
|
| Export Control Policy Training |
Self-Paced Online
|
| Patent Searching and Public Information Resources |
Self-Paced Online
|
| Foreign Influence – Heightened Awareness and Increased Activity |
Self-Paced Online
|
| Principles and Practices of Community-Based Research (CBR) at the University of Utah |
Self-Paced Online
|
| Effective Negotiation in Research: The Art of Advocacy and Agreement |
Self-Paced Online
|
| Clinical Research Coordinator (CRC) Orientation |
Self-Paced Online
|
| Predatory Journals |
Self-Paced Online
|
| Getting Published: Responsible Authorship and Peer Review - Faculty & Staff |
Self-Paced Online
|
| Clinical Research Budget Negotiation |
Self-Paced Online
|
| Budget Preparation and Development |
Self-Paced Online
|
| Pivot-RP: Funding Opportunities and Profiles |
Self-Paced Online
|
| Laboratory Leadership and Staffing |
Self-Paced Online
|
| Managing and Maintaining Your Scholarly Profile |
Self-Paced Online
|
| Rigor, Reproducibility, and Replicability in Research |
Self-Paced Online
|
| Use of a Compliance Toolkit |
Self-Paced Online
|
| Research Security Training |
Self-Paced Online
|
| EndNote Basic: Mastering EndNote Fundamentals for Citation Management |
Self-Paced Online
|
| Coverage Analysis Basics Certification |
Self-Paced Online
|
Research Administration (400-499)
| Budget Preparation and Development |
Live Online
|
| Proposals: How to Prepare and Submit |
Live Online
|
| Collaborative Research and the Roles of the Scientist in Society |
Live Online
|
| FDA Inspection 101 |
Live Online
|
| Access to the Utah Population Database |
Live Online
|
| Principles of Contracts, Subcontracts and SubAwards |
Self-Paced Online
|
| Proposals: How to Prepare and Submit |
Self-Paced Online
|
| Conflict of Interest (COI) |
Self-Paced Online
|
| Post-Award: Reporting & Closeout |
Self-Paced Online
|
IRB/Human Subjects (500-599)
| Data and Safety Monitoring: Plans, Boards & Committees |
Live Online
|
| Advanced Consideration of the Criteria for IRB Approval of Research |
Live Online
|
| Advanced Consideration of Reportable Events in Human Subject Research |
Live Online
|
| Informed Consent: Models and Requirements |
Live Online
|
| Preparation for Investigator-Initiated Drug and Device Studies |
Self-Paced Online
|
| Understanding IRB Applications in ERICA: New Studies, Amendments, and Continuing Review |
Self-Paced Online
|
| Multi-Center Research Using a Single Institutional Review Board (SIRB) |
Self-Paced Online
|
| Advanced Consideration of the Criteria for IRB Approval of Research |
Self-Paced Online
|
| Understanding IRB Applications in ERICA: Report Form |
Self-Paced Online
|
| Clinical Research at the Huntsman Cancer Institute |
Self-Paced Online
|
Modal Heading
Best Practice Networks (600-699)
REd Best Practice Networks offer a focused, one-hour forum for professional growth and peer exchange. Each session features a brief expert-led presentation followed by a collaborative discussion—an opportunity to explore ideas, share experiences, and strengthen campus practices.
Research Mentoring
| Laboratory Leadership and Staffing |
Not Available
|
| Topics in Mentoring and Mentee-ing for Grad/Undergrad Student and Post-Doctoral Scholars |
Not Available
|
Responsible Conduct of Research
| Ethics of Crowdsourcing Websites that Hire Workers for Research Projects |
Not Available
|
| Consent Document Simplification |
Not Available
|
| Academic Freedom and Authorship at the U of U |
Not Available
|
| Academic Freedom and Authorship at the U of U - Graduates & Postdocs |
Not Available
|
Compliance in Research Activities
| Unprofessional Behavior in the Research Environment |
Not Available
|
Finance & Budget Management
| Cayuse Proposals (424) Consultation |
Not Available
|
| Cost Share |
Coming Soon
|
| eAward - Guided Tour |
Not Available
|
| eProposal - Guided Tour |
Not Available
|
| Fastlane to Research.gov Transition |
Not Available
|
| Utilizing Startup Agreements for Clinical Trials |
Not Available
|
| Managing Risks with Contracts |
Not Available
|
Data Management
| Data Management in Clinical and Translational Research |
Not Available
|
| An Introduction to Using the University’s ELN, LabArchives |
Live Online
|
| How to Structure Your Data Collection |
Not Available
|
| National Research Data Resources |
Live Online
|
Cultural Awareness in Research Participation
| Cultural Awareness in Research Participation |
Live Online
|
Research Development
| PIVOT-RP Consultation |
Live Online
|
Special Topics
| Use of a Compliance Toolkit |
Not Available
|
| Broadening Impact: Data Basics for the Undergraduate Researcher |
Not Available
|
| Surrogate Consent by a Legally Authorized Representative |
Not Available
|
Research Education Certificate Programs (700-799)
Professional Behaviors Certificate
click here for full Certificate Details
| What is Research Professionalism? |
Self-Paced Online
|
| Professional Behaviors: NIH Focus |
Self-Paced Online
|
| Professional Behaviors: NSF Focus |
Self-Paced Online
|
| Professional Behaviors: Detrimental Research Practices |
Self-Paced Online
|
| Professional Behaviors: Mentorship |
Self-Paced Online
|
Research Mentoring Certificate
click here for full Certificate Details
| Introduction to Research Mentoring |
Self-Paced Online
|
| Establishing Expectations and Maintaining Effective Communication |
Live Online
|
| Assessing Understanding and Fostering Independence |
Live Online
|
| Fostering Academic Literacies |
Live Online
|
| Research and Mentoring Ethics |
Live Online
|
| Promoting Mentees’ Career Development |
Live Online
|
Responsible Conduct of Research (RCR) - Student Certificate
click here for full Certificate Details
| Foundations of Responsible Conduct of Research (RCR) - STUDENT |
Live Online
|
| Advanced Consideration of the Criteria for IRB Approval of Research OR |
Live Online
|
| Advanced Consideration of the Criteria for IRB Approval of Research |
Self-Paced Online
|
| Getting Published: Responsible Authorship and Peer Review - STUDENT |
Live Online
|
| Introduction to Research Mentoring |
Self-Paced Online
|
| Research Security Training |
Self-Paced Online
|
Responsible Conduct of Research (RCR) - Faculty & Staff Certificate
click here for full Certificate Details
| Foundations of Responsible Conduct of Research (RCR) - Faculty & Staff |
Self-Paced Online
|
| Case Studies in the Responsible Conduct of Research (RCR) |
Live Online
|
| Research Participant Advocacy and the Informed Consent Process |
Live Online
|
| Introduction to Research Mentoring |
Self-Paced Online
|
| Research Security Training |
Self-Paced Online
|
Compliance in Research Activities Certificate
click here for full Certificate Details
| Foundations in Research Compliance |
Self-Paced Online
|
| Source Documentation in Research OR |
Live Online
|
| Source Documentation in Research |
Self-paced Online
|
| Introduction to ClinicalTrials.gov |
Self-paced Online
|
| Expectations for Compliance in Human Subject Research |
Self-Paced Online
|
| Research Security Training |
Self-Paced Online
|
Finance & Budget Management in Research Certificate
click here for full Certificate Details
| Financial Management in Clinical Research OR |
Live Online
|
| Financial Management in Clinical Research |
Self-Paced Online
|
| Coverage Analysis (CA) |
Live Online
|
| Clinical Research Budget Development and the Importance of Clinical Research Billing Compliance |
Self-Paced Online
|
| Clinical Research Budget Oversight |
Self-Paced Online
|
| Budget Preparation and Development OR |
Self-Paced Online
|
| Budget Preparation and Development |
Live Online
|
| Clinical Research Budget Negotiation |
Self-Paced Online
|
Research Data Management Certificate
click here for full Certificate Details
| Introduction to Research Data Management |
Hybrid
|
Clinical Research Staff Foundations Certificate
click here for full Certificate Details
| Clinical Research Staff Foundations Certificate |
Self-Paced Online
|
Grant Writing (900-999)
Investigator Fundamentals
| Onboarding for New PIs: Submitting Proposals at the University of Utah |
Self-Paced Online
|
| Foundations/Corporate Funding |
Live Online
|
National Science Foundation (NSF)
Click here to learn about our VP-NSF Cohort Program
| National Science Foundation (NSF) Basics |
Self-Paced Online
|
| Grant Writing Workshop: The National Science Foundation (NSF) |
In Person
|
| Grant Writing Workshop: The National Science Foundation (NSF) Student Series |
In Person
|
| The National Science Foundation (NSF) - CAREER Program |
Self-Paced Online
|
National Institutes of Health (NIH)
| Science Experts Network Curriculum Vitae (SciENcv) |
Live Online
|
| Grant Writing Workshop - The National Institutes of Health (NIH) |
Self-Paced Online
|
Grant Writing Fundamentals
| Concision and Cutting Clutter |
Live Online
|
| Active Voice and Writing with Verbs |
Live Online
|
| Punctuation and Parallelism |
Live Online
|
| Communicating Research to General Audiences |
Live Online
|
| The Writing Process: Prewriting, Writing, and Editing |
Live Online
|
| Crafting Focused Paragraphs |
Live Online
|
| Introductions and Literature Reviews |
Live Online
|
Not with the University of Utah?
Certificate programs and research education courses are available from the Office of Research Education to individuals outside the University of Utah community. Visit the Academy of Research Education website to learn more.
RED Classes FAQ
-
Go to the Research Education website homepage.
-
On the red menu bar, there are several ways to search for classes:
-
Certificates: View certificate program details, instructions, and required classes
- Class Catalog: Browse the full REd curriculum, organized by category

-
- Select the class you would like to enroll in. This will take you to the Catalog listing for that class.

-
Once in Catalog, select “Login” in the top right corner.
If you are University of Utah Employee or Student, sign in to Catalog with your uNID and password.
If you are not affiliated with University of Utah, please visit the Academy of Research Educationfor available classes.
-
Once logged in, select the course listing.
- Select “Enroll Now”.

-
Once you are enrolled, you will be able to access the class through your Canvas Catalog dashboard or your Canvas dashboard.
- Canvas Catalog dashboard.
Email confirmation
- Go to Research Education Canvas Catalog and click on "Student Dashboard" under your name.

- To access your class through the Canvas dashboard
Go to https://cis.utah.edu/
Click on "Canvas Login" under either Employee or Student Portal
Click on the class you enrolled in
- Canvas Catalog dashboard.
If you can no longer attend a live class or would no longer like to be enrolled in an online class, please follow below steps to drop the class from your Student Dashboard on Catalog.
Access the Research Education Canvas Catalog at the link below
Research Education Canvas Catalog
or by clicking on any class listing on our site

After you enter the Research Education Canvas Catalog, please follow below steps to drop the class:
-
Login at the top right corner of the page.
-
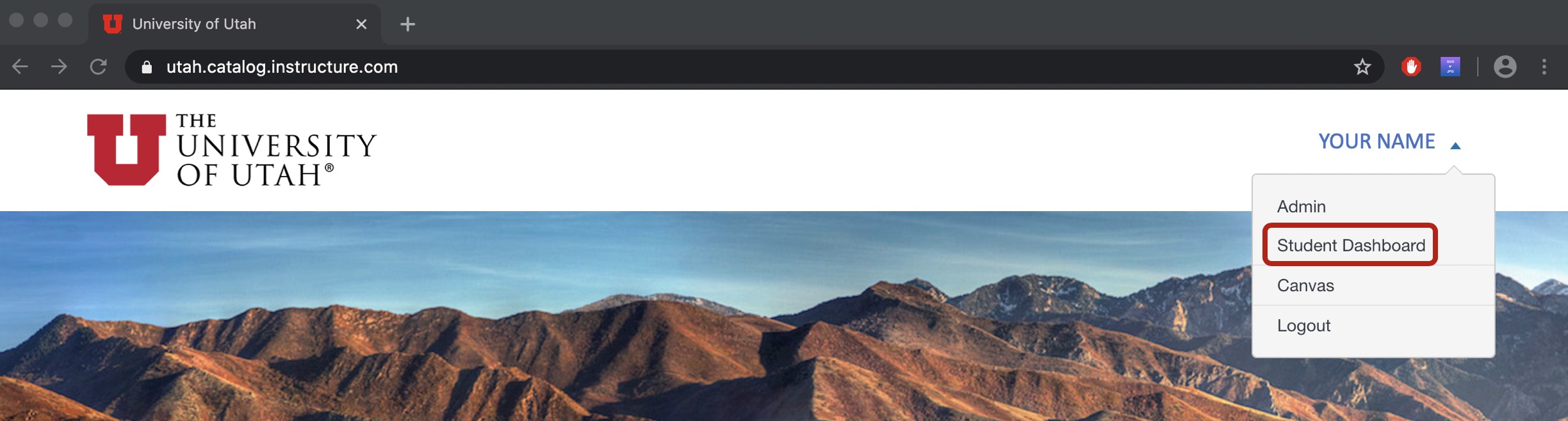
Login by using your uNID and password, then you should see your Canvas Catalog dashboard. If not, you can click on your name on the top right of the page and select Student Dashboard.
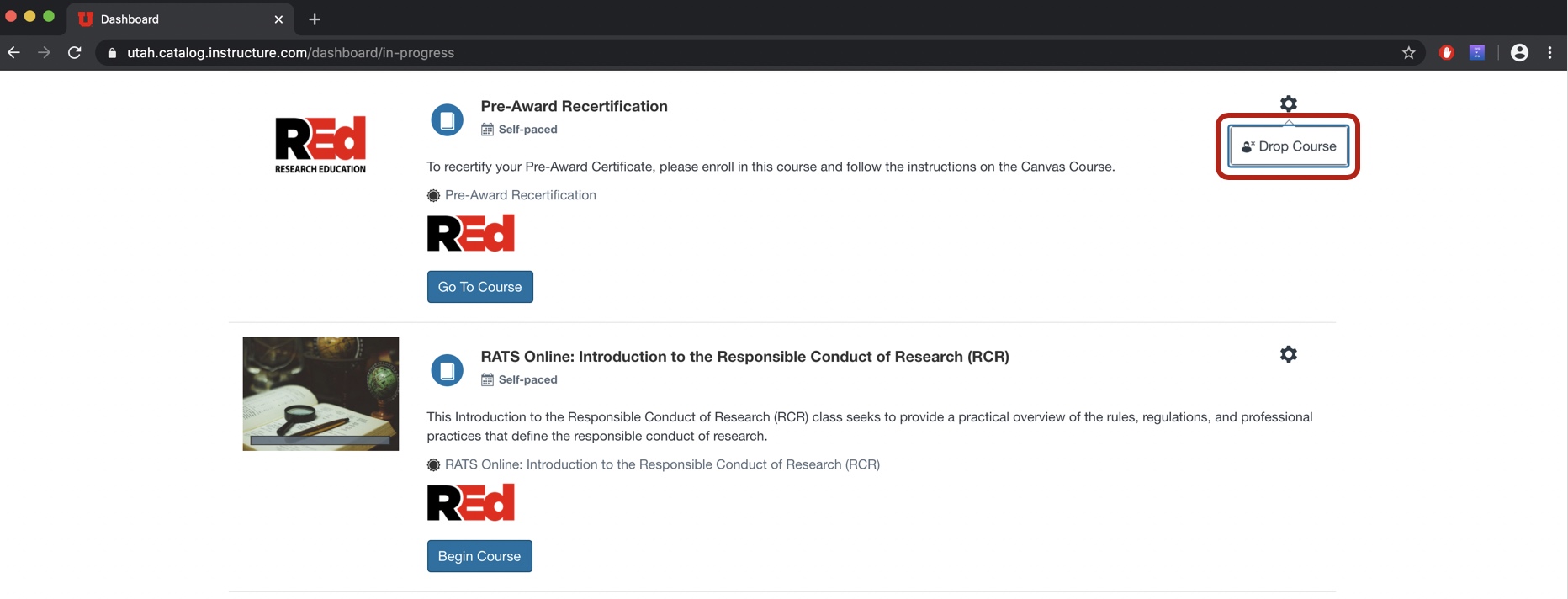
- Find the class that you want to drop, click on the gear icon (
 ) and select Drop Course.
) and select Drop Course.